Efficacy of Intravitreal Anti Vascular Endothelial Growth Factor Injection Compared to Focal Therapy in Pediatric Patients with Coats Disease
DOI:
https://doi.org/10.35749/journal.v48i2.100602Keywords:
anti-VEGF, Coats disease, focal therapyAbstract
Introduction: Coats disease is a retinal vascular disorder that may lead to progressive exudative retinal detachment. The standard regiment of treatment is focal therapy. However, its effectiveness may decrease when more than two quadrants of the retina exhibit vascular abnormalities. Thus, recent studies tried to use anti vascular endothelial growth factor (anti-VEGF) as therapy for the vascular problems.
Aims: Comparing the efficacy between anti-VEGF injection and standard therapy regiment such as focal laser therapy.
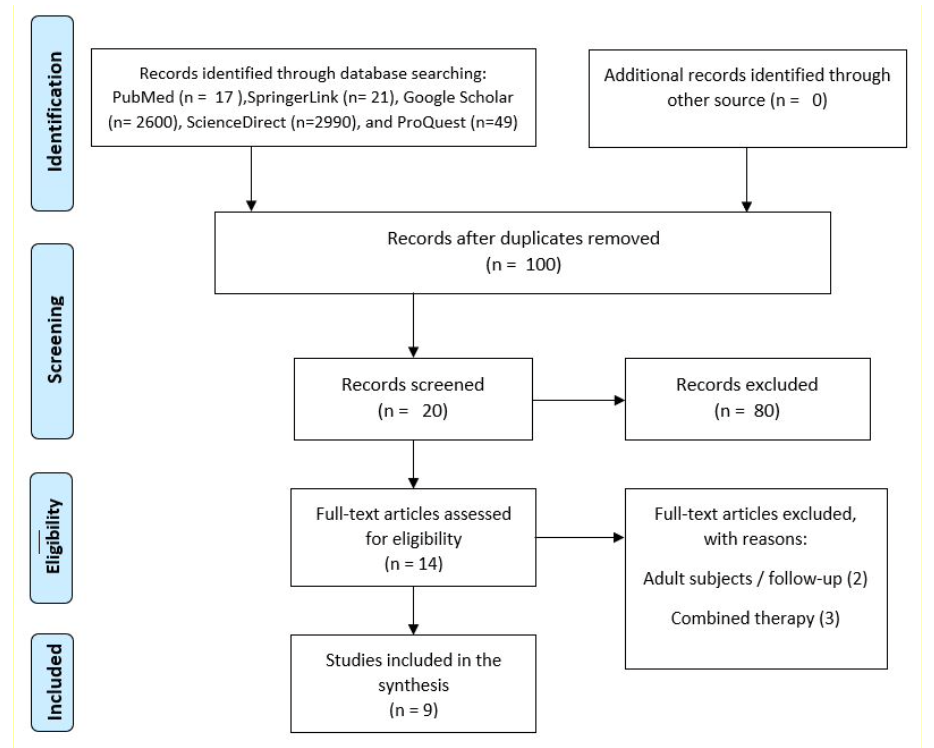
Methods: Literature searching was conducted through PubMed, ScienceDirect, Google Scholar, ProQuest, and SpringerLink. Search terms included "Coats’ Disease" and mesh terms of “Anti- VEGF". The efficacy is assessed based on Best-Corrected Visual Acuity (BCVA) and improvement in fundus manifestations.
Results: All of the studies that conducted primary intravitreal anti-VEGF treatment were followed by necessary ablative treatment. These studies, such as by Yang Q and Zheng XX, et al. showed remarkable improvements in both visual acuity and anatomical outcomes. Laser therapy also gained satisfactory result in upgrading clinical stages, even though some complications including subretinal fibrosis, cataract, and vitreous hemorrhage were reported. A comparative study by Ray R, et al. noted that group undergoing anti-VEGF might require longer treatment sessions despite possible benefits.
Conclusion: Anti-VEGF agents can be used as neoadjuvant in standard therapy. It did not reduce the time for full treatment, but the resolution of disease was seen in the most severe cases treated with combination therapy.
Downloads
References
Ray R, Barañano DE, Hubbard GB. Treatment of Coats’ disease with intravitreal bevacizumab. Br J Ophthalmol. 2013;97(3):272-277. doi:10.1136/bjophthalmol-2012-302250
Morris B, Foot B, Mulvihill A. A population-based study of Coats disease in the United Kingdom I: Epidemiology and clinical features at diagnosis. Eye. 2010;24(12):1797-1801. doi:10.1038/eye.2010.126
Irfani I, Kartasasmita AS. Pediatric Retinal Detachment in Indonesia: Clinical Characteristics, Risk Factors, and Treatment Outcomes. Open J Ophthalmol. 2017;07(04):249-255. doi:10.4236/ojoph.2017.74033
Sigler EJ, Randolph JC, Calzada JI, Wilson MW, Haik BG. Current management of Coats disease. Surv Ophthalmol. 2014;59(1):30-46. doi:10.1016/j.survophthal.2013.03.007
Mrejen S, Metge F, Denion E, Dureau P, Edelson C, Caputo G. Management of retinal detachment in coats disease: Study of 15 cases. Retina. 2008;28(3 SUPPL.):26-32. doi:10.1097/IAE.0b013e31816b3158
Char DH. Coats ’ syndrome : long term follow up. Br J Ophthalmol. 2000;84:37-39.
Zhao Q, Peng XY, Chen FH, et al. Vascular endothelial growth factor in Coats’ disease. Acta Ophthalmol. 2014;92(3):225-228. doi:10.1111/aos.12158
Ramasubramanian A, Shields CL. Bevacizumab for Coats’ disease with exudative retinal detachment and risk of vitreoretinal traction. Br J Ophthalmol. 2012;96(3):356-359. doi:10.1136/bjophthalmol-2011-300141
Lin CJ, Hwang JF, Chen YT, Chen SN. The effect of intravitreal bevacizumab in the treatment of coats disease in children. Retina. 2010;30(4):617-622. doi:10.1097/IAE.0b013e3181c2e0b7
Yang Q, Wei W, Shi X, Yang L. Successful use of intravitreal ranibizumab injection and combined treatment in the management of Coats’ disease. Acta Ophthalmol. 2016;94(4):401-406. doi:10.1111/aos.13067
Zheng XX, Jiang YR. The effect of intravitreal bevacizumab injection as the initial treatment for Coats’ disease. Graefe’s Arch Clin Exp Ophthalmol. 2014;252(1):35-42. doi:10.1007/s00417-013-2409-1
Li S, Deng G, Liu J, Ma Y, Lu H. The effects of a treatment combination of anti-VEGF injections, laser coagulation and cryotherapy on patients with type 3 Coat’s disease. BMC Ophthalmol. 2017;17(1):1-7. doi:10.1186/s12886-017-0469-4
Shapiro MJ, Chow CC, Karth PA, Kiernan DF, Blair MP. Effects of green diode laser in the treatment of pediatric coats disease. Am J Ophthalmol. 2011;151(4):725-731.e2. doi:10.1016/j.ajo.2010.10.024
Mulvihill A, Morris B. A population-based study of Coats disease in the United Kingdom II: Investigation, treatment, and outcomes. Eye. 2010;24(12):1802-1807. doi:10.1038/eye.2010.127
Levinson JD, Hubbard GB. 577-NM yellow laser photocoagulation for coats disease. Retina. 2016;36(7):1388-1394. doi:10.1097/IAE.0000000000000874
Grosso A, Pellegrini M, Cereda MG, Panico C, Staurenghi G, Sigler EJ. Pearls and pitfalls in diagnosis and management of coats disease. Retina. 2015;35(4):614-623. doi:10.1097/IAE.0000000000000485
Böhm MRR, Uhlig CE. Use of intravitreal triamcinolone and bevacizumab in Coats’ disease with central macular edema. Graefe’s Arch Clin Exp Ophthalmol. 2011;249(7):1099-1101. doi:10.1007/s00417-011-1629- 5
He YG, Wang H, Zhao B, Lee J, Bahl D, McCluskey J. Elevated vascular endothelial growth factor level in Coats’ disease and possible therapeutic role of bevacizumab. Graefe’s Arch Clin Exp Ophthalmol. 2010;248(10):1519-1521. doi:10.1007/s00417-010-1366-1
Cebeci Z, Bayraktar ?, Y?lmaz YC, Tuncer S, K?r N. Evaluation of follow-up and treatment results in coats’ disease. Turk Oftalmoloiji Derg. 2016;46(5):226-231. doi:10.4274/tjo.12754
Arevalo JF, Maia M, Flynn HW, et al. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92(2):213-216. doi:10.1136/bjo.2007.127142
Villegas VM, Gold AS, Berrocal AM, Murray TG. Advanced Coats’ disease treated with intravitreal bevacizumab combined with laser vascular ablation. Clin Ophthalmol. 2014;8:973-976. doi:10.2147/OPTH.S62816
Zhang L, Ke Y, Wang W, Shi X, Hei K, Li X. The efficacy of conbercept or ranibizumab intravitreal injection combined with laser therapy for Coats’ disease. Graefe’s Arch Clin Exp Ophthalmol. 2018;256(7):1339-1346. doi:10.1007/s00417-018-3949-1
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2022 Alia Nesa Utami, Julie Dewi Barliana

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
