A RARE CASE OF SUBCONJUNCTIVAL HEMORRHAGE POST COVID-19 mRNA VACCINE: A CASE REPORT
A Rare Case of Subconjunctival Hemorrhage Post COVID-19 mRNA Vaccine: A Case Report
DOI:
https://doi.org/10.35749/journal.v49i2.100699Keywords:
subconjunctival hemorrhage, AEFI, COVID-19, mRNA vaccineAbstract
Introduction: Concurrently with the administration of COVID-19 vaccine, adverse event following immunization (AEFI) began to be reported. Some reactions involve various systems of the human body, including the ocular system. Although uncommon, subconjunctival hemorrhage also can be found, as shown in this report.
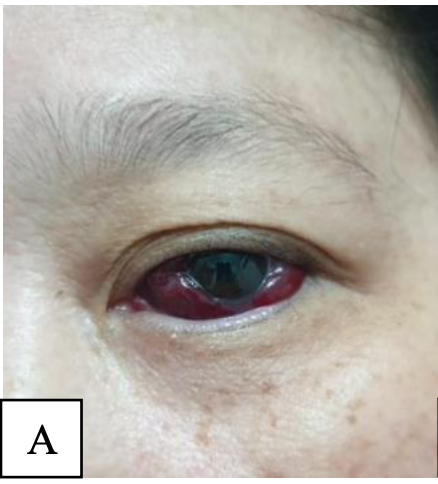
Case Report: We present a case of a 68-year-old woman with subconjunctival hemorrhage a couple hours before admission. Patient also came with complaints of sudden redness and pain in left eye. Two days prior, she had her first dose of Moderna vaccine. Left eye examination revealed hemorrhage on conjunctiva with normal visual acuity. Other ocular examinations couldn’t be done due to the lack of facilities and severe pain. No other symptoms were mentioned. Patient was advised to be referred to an ophthalmologist, but she refused. After a month, the patient reportedly experienced similar complaints after receiving the second dose of Moderna vaccine. another redness and discomfort in her left eye. Her left eye was red, itched, and swollen. However, she still refused to go to an ophthalmologist. Oral analgesic, oral antihistamine, and artificial tears were given. The symptoms were completely resolved in a couple days.
Conclusion: Subconjunctival hemorrhage may occur as mRNA Vaccine AEFI specifically in patients with underlying disease, however further research is needed.
Downloads
References
Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157–60
Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022; 22: 1293–302
Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Moderna COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69(5152):1653–6.
El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, et al. Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N Engl J Med. 2021;385(19):1774–85.
Self WH, Tenforde MW, Rhoads JP, Gaglani M, Ginde AA, Douin DJ, et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions — United States, March–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(38):1337-1343
Sodhi M, Etminan M. Reported ocular adverse events with three COVID-19 vaccines. Acta Ophthalmol. 2022;100(7):e1537-e1538.
Hu D-N, Mou C-H, Chao S-C, Lin C-Y, Nien C-W, Kuan P-T, et al. Incidence of Non-Traumatic Subconjunctival Hemorrhage in a Nationwide Study in Taiwan from 2000 to 2011. PLoS ONE. 2015; 10(7): e0132762
Tarlan B, Kiratli H. Subconjunctival hemorrhage: risk factors and potential indicators. Clinical Ophthalmology. 2013;7: 1163–1170
Doshi R, Noohani T. Subconjunctival Hemorrhage [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 [updated 2022 Feb 23; cited 2022 Sep 15]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551666/
Fukuyama J-I, Hayasaka S, Yamada K, Setogawa T. Causes of Subconjunctival Hemorrhage. Ophthalmologica. 1990; 200:63-67
Medicine and Healthcare Product Regulatory Agency (GB). COVID-19 Moderna Vaccine Analysis Print [Internet]. London: Medicine and Healthcare Product Regulatory Agency; 2022 [updated 2022 Aug 25, cited 2022 Sep 10]. Available from:
Supangat, Sakinah EN, Nugraha MY, Qodar TS, Mulyono BW, Tohari AI. COVID-19 Vaccines Programs: adverse events following immunization (AEFI) among medical Clerkship Student in Jember, Indonesia. BMC Pharmacology and Toxicology. 2021;22:58
Testi I, Brandão-de-Resende C, Agrawal R, Pavesio C, COVID-19 Vaccination Ocular Inflammatory Events Study Group. Ocular inflammatory events following COVID-19 vaccination: a multinational case series. J Ophthalmic Inflamm Infect. 2022;12(1):4
Park J, Lagniton P, Liu Y, Xu R. mRNA vaccines for COVID-19: what, why and how. International Journal of Biological Sciences. 2021;17(6):1446-1460.
Park H, Byun Y, Byeon S, Kim S, Kim Y, Lee C. Retinal Hemorrhage after SARS-CoV-2 Vaccination. Journal of Clinical Medicine. 2021;10(23):5705.
Ng X le, Betzler BK, Ng S, Chee SP, Rajamani L, Singhal A, et al. The eye of the storm: COVID-19 vaccination and the eye. Ophthalmol Ther. 2021;1–20.
Amin MA, Nahin S, Dola TA, Afrin S, Hawlader MDH. Retinal hemorrhage of late post?COVID?19 and post?vaccine?related pathogenic mechanisms: A new challenge for ophthalmologist in COVID era. Clin Case Rep. 2022;10(2):e05471.
Sodhi PK, Jose R. Subconjunctival hemorrhage: the first presenting clinical feature of idiopathic thrombocytopenic purpura. Jpn J Ophthalmol. 2003;47(3):316–8.
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2023 Jovita Jutamulia, Arlin Chyntia Dewi, Salma Salsabila, Vicky Octaviani

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.